Non-Migratory Plasticizers, including Phthalate Mimics
Phthalate plasticizers are currently used on the million tons / year scale to convert inherently brittle polyvinyl chloride to form a wide variety of heat-extrudable, pliable consumer products. The most common phthalate plasticizer is DEHP:
Development of a substitute for Phthalate Plasticizers
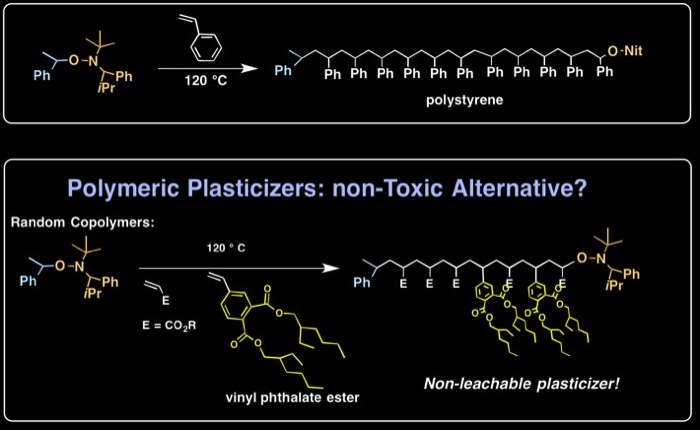
Our first approach used Nitroxide Mediated Polymerization (NMP), which is regularly carried out using styrene. We reasoned that vinyl phthalate esters should behave as substituted styrenes in NMP. We demonstrated that a variety of macromolecular plasticizers can be produced, as both homopolymers and random copolymers.
R. Braslau,* F. Schäffner, A. Earla “Polymeric phthalates: potential non-migratory macromolecular plasticizers,” Journal of Polymer Science Part A: Polymer Chemistry, 2013, 51, 1775-1184. doi: 10.1002/pola.26485.
• Polymerization of Vinyl Phthalate Esters to form Macromolecular Plasticizers
• Triazole Analogues of Phthalate Plasticizers covalently linked to PVC

Traditional plasticizers are mixed with granular PVC and melted into a matrix of PVC and other additives, from which building materials, clothes, food packaging, and medical supplies (ex. blood bags) are produced. The phthalates migrate out of these commodity products: if absorbed or ingested, they are metabolized to form endocrine signaling compounds, resulting in a variety of diseases including cancer, sexual malformation, and obesity.
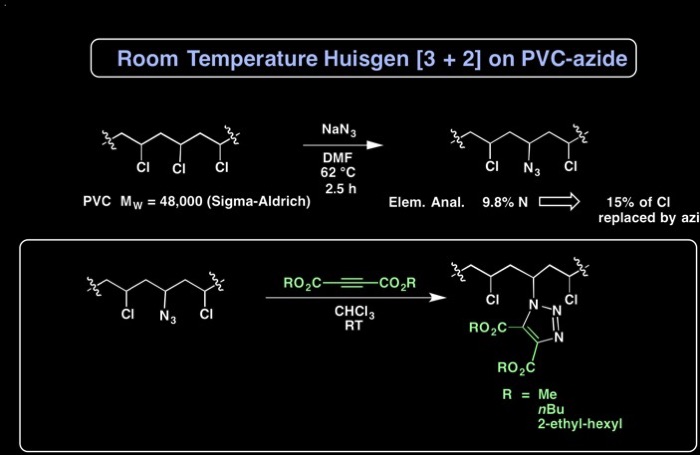
Looking for an inexpensive method to attach phthalate plasticizers to PVC, we envisioned that a "click" reaction between an azide and acetylene dicarboxylates would form triazoles bearing ortho esters, as phthalate mimics covalently attached to PVC. This copper-free click reaction between azide and acetylene dicarboxylates occurs under mild conditions, making this attractive to industry.
A. Earla, L. Li, P. Costanzo, R. Braslau “Phthalate Plasticizers Covalently Linked to PVC via Copper-Free or Copper Catalyzed Azide-Alkyne Cycloadditions,” Polymer, 2017, 109, 1-12.
DOI: 10.1016/j.polymer.2016.12.014
A. Earla, R. Braslau* “Covalently Linked Plasticizers: Triazole Analogues of Phthalate Plasticizers Prepared by mild Copper-free “Click” Reactions with Azide-functionalized PVC,” Macromolecular Rapid Communications, 2014, 35, 666-671. doi: 10.1002/marc.201300865.
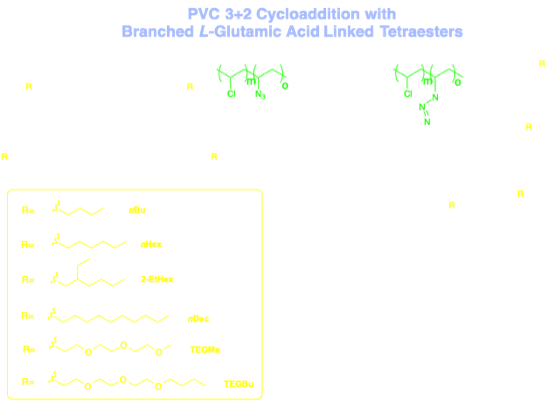
We also explored attaching four plasticizing groups per azide on the PVC via a glutamic acid linkage:
Longbo Li, Andy T. Tek, Rudy J. Wojtecki, and Rebecca Braslau,* “Internal Plasticization of Poly(vinyl) Chloride using Glutamic Acid as a Branched Linker to Incorporate Four Plasticizers per Anchor Point,” Journal of Polymer Science Part A: Polymer Chemistry, 2019, 57, 1821-1835. DOI: 10.1002/pola.29455
.2 We then explored a variety of approaches using this mild thermal Huisgen cycloaddition. As the structure of the plasticizer DEHP can be drawn to resemble a "frog," we represent these covalent plasticizers as Frogs or Tadpoles, Two Frogs on Frog, or Frog on Tadpole. The key finding is that esters bearing long PEG groups are extremely effective plasticizers, and propargylic esters (cheaper than acetyleneic diesters) bearing PEG groups are great internal plasticizers when attached to PVC via a triazole linkage in the "Tadpole" formulation.
Chad M. Higa, Andy T. Tek, Rudy J. Wojtecki, Rebecca Braslau,* "Non-Migratory Internal Plasticization of Poly(Vinyl Chloride) via Pendant Triazoles Bearing Alkyl or Polyether Esters" Journal of Polymer Science Part A: Polymer Chemistry, 2018, 56, 2397-2411. DOI: 10.1002/pola.29205

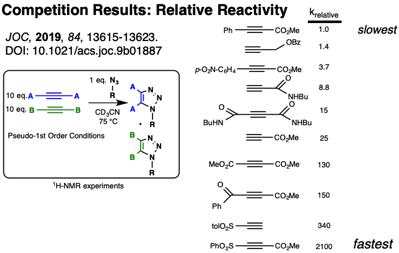
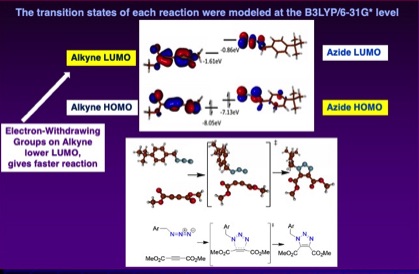
As an offshoot to this research, the relative reactivity of electron poor alkynes was investigated by comparing relative reactivities in competition experiments with a representative organoazide, as well as by modeled at the B3LYP/6-31G* level:
Patrick W. Skelly, Jirapon Sae-Jew, Ana Paula Kitos Vasconcelos, Jerin Tasnim, Longbo Li, Jevgenij Raskatov, and Rebecca Braslau,* “Relative Rates of Metal-Free Azide-Alkyne Cycloadditions: Tunability over Three Orders of Magnitude,” Journal of Organic Chemistry, 2019, 84, 13615-13623.
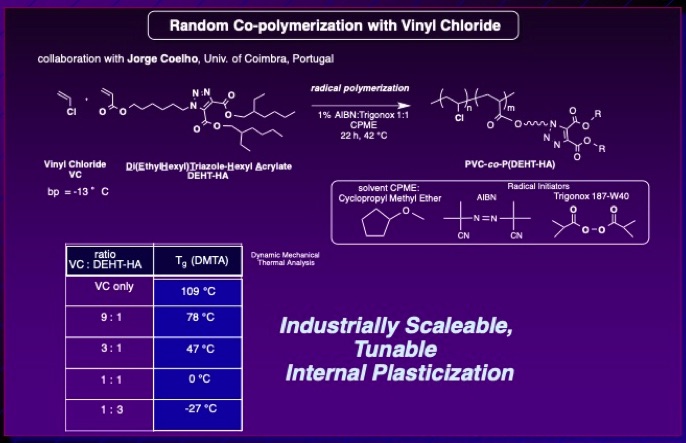
As a collaboration with Jorge Coelho and Armenio Serra at the University of Coimbra in Portugal (where they have the equipment and expertise to safely handle vinyl chloride!):
Talita C. Rezende, Carlos M. R. Abreu, Ana C. Fonseca, Chad M. Higa, Longbo Li, Armenio C. Serra, Rebecca Braslau and Jorge F. J. Coelho “Efficient Internal Plasticization of Poly(Vinyl Chloride) via Free Radical Copolymerization of Vinyl Chloride with an Acrylate Bearing a Triazole Phthalate Mimic,” Polymer, 2020, 196, 122473-122481. DOI: 10.1016/j.polymer.2020.122473
• Random Copolymerization of Plasticizing Monomers with Vinyl Chloride
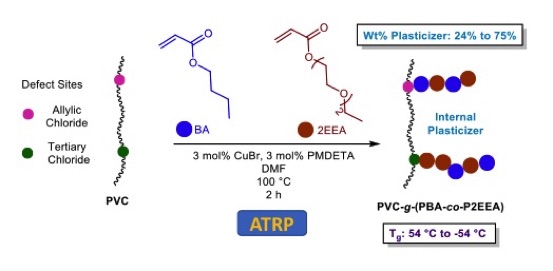
• ATRP to Grow Graft Copolymers from Defect Sites in PVC
PVC is made by conventional (uncontrolled) free radical polymerization, and inherently contains both allylic and tertiary chloride defect sites. Building on foundational work by Percec, we have found that the use of acrylates (rather than methacrylates) can be used to grow effective internal plasticizing grafts onto PVC as a single step from commercial PVC:
Longbo Li, Yanika Schneider, Adrienne B. Hoeglund, Rebecca Braslau,* “Advances in Internal Plasticization of PVC: Copper-Mediated Atom Transfer Radical Polymerization From PVC Defects Sites to Form Acrylate Graft Copolymers,” Synlett, 2021, 32, 497-501. DOI: 10.1055/s-0037-1610764
Longbo Li, Yanika Schneider, Adrienne B. Hoeglund, Rebecca Braslau,* “Internal Plasticization of Poly(Vinyl Chloride) by Grafting Acrylate Copolymers via Copper-Mediated Atom Transfer Radical Polymerization,” J. Appl. Poly. Sci., 2021, 138, 50747. DOI: 10.1002/app.50747