Synthetic Methodology Development
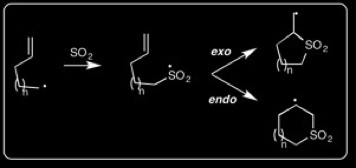
We have developed a convergent [n+1] radical annulation using SO2 to prepare 5-7-membered cyclic sulfones in one step. This strategy is quite novel; the substrates are easily prepared, and the reaction proceeds under very mild conditions. We can control the endo vs. exo regioselectivity by preparing either branched or linear allylic sulfide terminating groups, respectively: A. Tsimelzon, R. Braslau* "N+1 Radical Annulations with SO2," Journal of Organic Chemistry, 2005, 70, 10854-10859.
Radical [n+1] Cyclizations with SO2
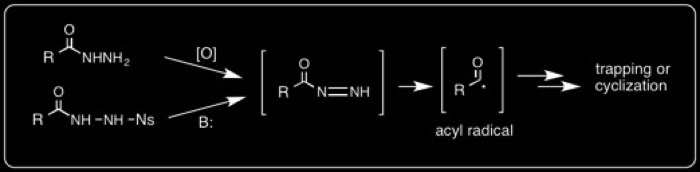
In another synthetic methodology project, we have developed a method to stoichiometrically generate acyl radicals from acyl hydrazines.
• Acyl Radical Generation and Cyclizations
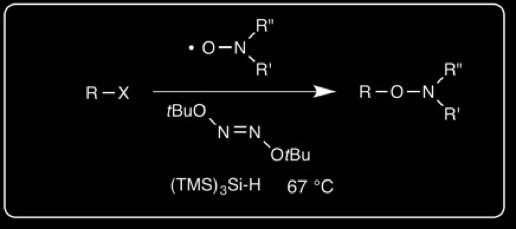
• Synthesis of N-Alkoxyamines
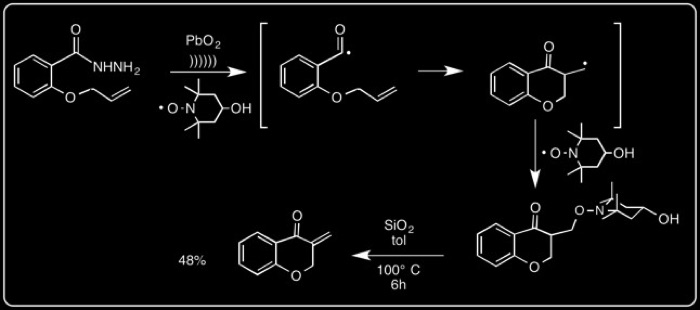
The mechanism is illustrated below with an example of a [5+1] 6-endo-trig cyclization.
We have developed a preparation of N-alkoxyamines by silyl radical abstraction from alkyl halide precursors. This is particularly valuable for preparing alkoxyamines in which R is a tertiary alkyl group.
R. Braslau,* A. Tsimelzon, and J. Gewandter “A Novel Methodology for the Synthesis of N-Alkoxyamines” Organic Letters, 2004, 6, 2233-2235.
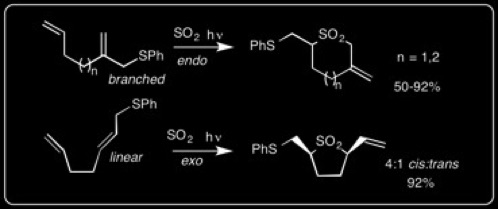
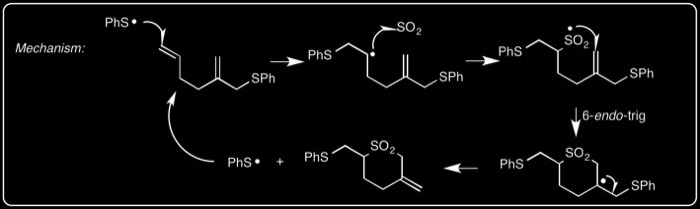
These can be used in 5-exo and 6-exo cyclization reactions: R. Braslau,* M. O. Anderson, F. Rivera, T. Haddad, A. Jimenez, and J. R. Axon, “Acyl Hydrazines as Precursors to Acyl Radicals” Tetrahedron, 2002, 58, 5513-5523.